‘Artificial ovary’ allows human eggs to be matured outside the body
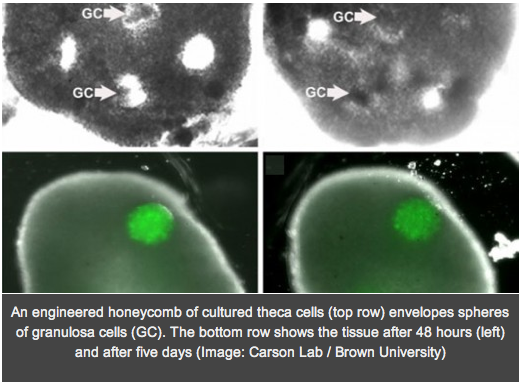 In a move that could yield infertility treatments for cancer patients and provide a powerful new means for conducting fertility research, researchers have built an artificial human ovary that can grow oocytes into mature human eggs in the laboratory. The ovary not only provides a living laboratory for investigating fundamental questions about how healthy ovaries work, but also can act as a testbed for seeing how problems, such as exposure to toxins or other chemicals, can disrupt egg maturation and health. It could also allow immature eggs, salvaged and frozen from women facing cancer treatment, to be matured outside the patient in the artificial ovary.
In a move that could yield infertility treatments for cancer patients and provide a powerful new means for conducting fertility research, researchers have built an artificial human ovary that can grow oocytes into mature human eggs in the laboratory. The ovary not only provides a living laboratory for investigating fundamental questions about how healthy ovaries work, but also can act as a testbed for seeing how problems, such as exposure to toxins or other chemicals, can disrupt egg maturation and health. It could also allow immature eggs, salvaged and frozen from women facing cancer treatment, to be matured outside the patient in the artificial ovary.To create the ovary, the researchers at Brown University and Women & Infants Hospital formed honeycombs of theca cells, one of two key types of cells in the ovary, donated by reproductive-age (25-46) patients at the hospital. After the theca cells grew into the honeycomb shape, spherical clumps of donated granulosa cells were inserted into the holes of the honeycomb together with human egg cells, known as oocytes. In a couple days the theca cells enveloped the granulosa and eggs, mimicking a real ovary. In experiments the structure was able to nurture eggs from the “early antral follicle” stage to mature human eggs.
Sandra Carson, professor of obstetrics and gynecology at the Warren Alpert Medical School of Brown University and director of the Division of Reproductive Endocrinology and Infertility at Women & Infants Hospital, said her goal was never to invent an artificial organ, per se, but merely create a research environment in which she could study how theca and granulose cells and oocytes interact. She then heard of the so-called “3D Petri dishes” developed by Jeffrey Morgan that are made of a moldable agarose gel that provides a nurturing template to encourage cells to assemble into specific shapes. The two then teamed up to create the organ, resulting in the first fully functioning tissue to be made using Morgan’s method.
The paper detailing the development of the artificial ovary appears in the Journal of Assisted Reproduction and Genetics.
